BCR-ABL1 oncogene testing
What is tested in this type of analysis?
The analysis focuses on detecting the presence and quantity of the BCR-ABL1 oncogene, a fusion gene formed by the translocation of genetic material between chromosomes 9 and 22. This gene fusion is commonly associated with chronic myeloid leukaemia (CML) and a subset of acute lymphoblastic leukaemia (ALL).

What do the results indicate?
A positive result indicates the presence of the BCR-ABL1 oncogene, confirming the diagnosis of CML or Ph-positive ALL. The quantity of BCR-ABL1 transcripts detected in the sample can provide valuable information about disease progression and response to treatment.
Why should the test be performed?
This test is essential for diagnosing CML and Ph-positive ALL, guiding treatment decisions, monitoring disease progression, and assessing treatment response. Early detection and monitoring of BCR-ABL1 levels can help optimise therapy and improve patient outcomes.
When should the test be conducted?
The analysis should be conducted at diagnosis to confirm the presence of the BCR-ABL1 oncogene. Subsequent monitoring of BCR-ABL1 levels is performed at regular intervals during treatment to assess treatment response and disease progression.
What type of sample is required?
A blood sample is typically required for BCR-ABL1 oncogene testing. Peripheral blood or bone marrow aspirate samples may be used, depending on the patient's clinical presentation and stage of disease.
Is any prior preparation necessary?
No specific prior preparation is necessary for the analysis. However, patients may be advised to follow certain instructions provided by their healthcare provider regarding fasting or discontinuation of certain medications, if applicable.
How is the test sample used?
The analysis involves molecular techniques such as polymerase chain reaction (PCR) or fluorescent in situ hybridisation (FISH) to detect and quantify BCR-ABL1 transcripts. Results are interpreted by healthcare professionals to guide treatment decisions and monitor disease progression.
What are the normal values?
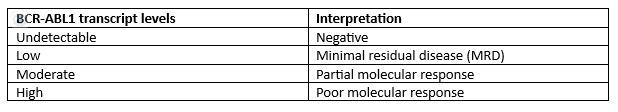
What do altered values indicate?
Elevated BCR-ABL1 transcript levels indicate active disease or treatment resistance, requiring reassessment of treatment strategies. Conversely, declining or undetectable BCR-ABL1 levels suggest a favourable treatment response and disease control.